Renal acid-base handling
Non-volatile acids (i.e. acids that aren't CO2) cannot be eliminated by the lungs. They therefore must rely on the kidneys for elimination (e.g. lactate, ketones, sulfates, phosphates).
The basic principle is that an acid load causes a bicarbonate deficit. Kidneys handle this acid load by excreting acids and replacing the bicarbonate deficit.
Acid-base handling along the nephron
Glomerulus
Bicarbonate is freely filtered at the glomerulus.
pH is 7.4 at the glomerulus.
Proximal convoluted tubule (PCT)
80% of bicarbonate is reabsorbed.
In PCT cells
- H2O + CO2 → bicarbonate + H+ (via carbonic anhydrase)
- bicarbonate is reabsorbed with Na/HCO3- co-transporters
- H+ is secreted into the lumen in exchange for Na via sodium-hydrogen antiporter 3 (NHE3)
In the lumen
- the H+ that was secreted into the lumen via the above process combines with filtered bicarbonate:
- bicarbonate + H+ → CO2 + H2O (via carbonic anhydrase)
- CO2 that is produced in the lumebn by this reaction diffuses into PCT cells to fuel the production of more bicarbonate
The net effect
- filtered bicarbonate is preserved and reabsorbed
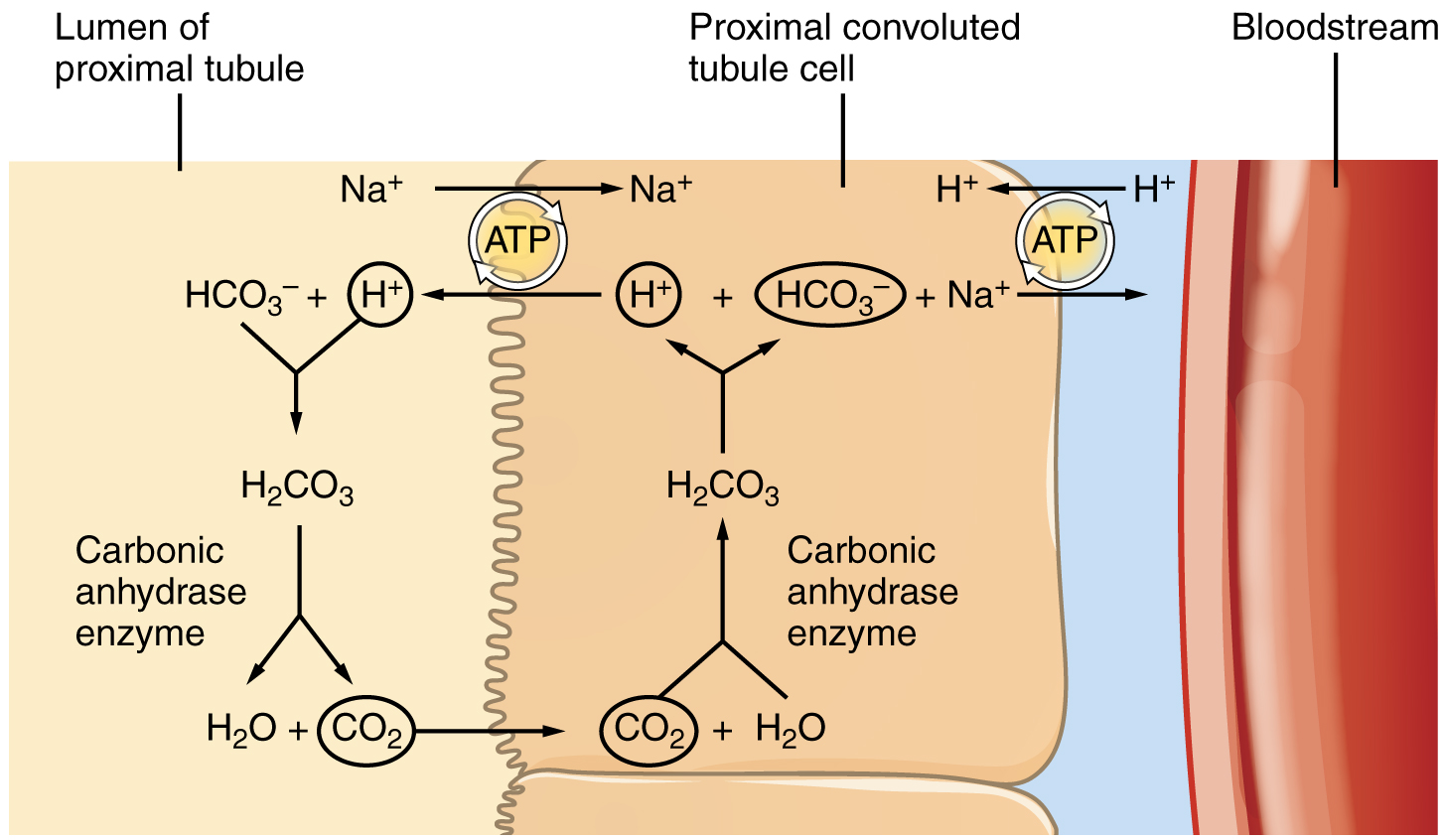
Bicarbonate reabsorption in the PCT
By OpenStax College, licensed under CC BY 3.0
Loop of Henle
An additional 10% of bicarbonate is reabsorbed.
NHE3 transporters facilitate further H+ secretion.
Distal nephron
The minimum urine pH is 4.5 in the collecting ducts.
Type A intercalated cells (aka alpha-intercalated cell)
- in luminal cells
- H2O + CO2 → bicarbonate + H+ (via carbonic anhydrase)
- apical H-ATPase and H-K-ATPase pumps → H+ secretion
- basolateral bicarbonate-chloride antiporter → bicarbonate reabsorption
- in lumen
- secreted H+ binds to non-bicarbonate urinary buffers (e.g. phosphates and ammonium) as most luminal bicarbonate has already beed reabsorbed
- this allows acid secretion without loss of bicarbonate
- net effect
- bicarbonate is created
- H+ is excreted
Type B intercalated cells (aka beta-intercalated cell)
- structurally the same as type A intercalated cells however the apical and basolateral membranes are reversed
- facilitates secretion of bicarbonate and reabsorption of H+
Regulation
- in response to an acid load
- type A cells → increased number transporters on both membranes → favours bicarbonate reabsoption and H+ secretion
- in response to a base load
- type B cells → increased number transporters on both membranes → favours bicarbonate secretion and H+ reabsorption
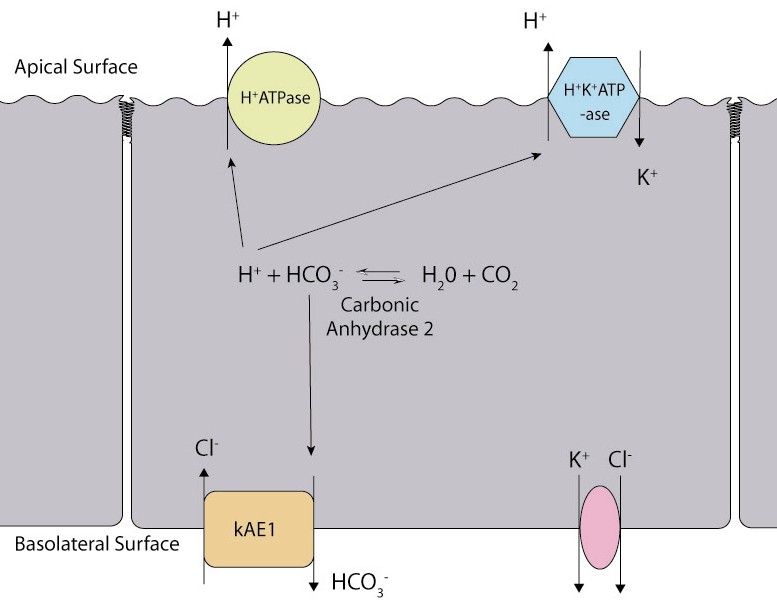
Acid-base handling in type A intercalated cells
Describe the renal buffer systems
Renal buffer systems allow for the conservation of bicarbonate and excretion of H+ in the distal nephron.
Phosphate buffer system
- pKa 6.8
- H2PO4- is a weak acid
- HPO42- is a weak base
- 80% filtered of phosphate is in weak base form (as serum pH is 7.4)
Mechanism
- H+ secretion in the distal nephron shifts this equation leftward, binding HPO42- and forming the weak acid H2PO4- which is excreted.
- hence this process allows the excretion of H+
- this is known as titrated acid secretion
- acidosis leads to increased acid secretion which increases the effectiveness of the phosphate buffer
Capacity
- The filtered load of phosphate is 160mmol/day
- 75% is reabsorbed
- therefore 40mmol/day of acid can be secreted by the phosphate buffer system
Limitations
- Phosphate may bind other cations (Ca2+, Mg2+, Na+) which limits its buffering capacity
- This system has limited ability to adapt to acidaemia or alkalaemia, which generally relies on ammoniagenesis
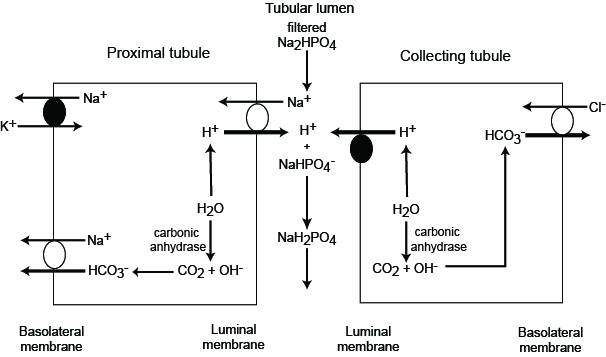
The phoshate buffer system
By eClinPath (Cornell University), licensed under CC-BY-NC-SA 4.0
Ammonium buffer system
- pKa 9.3
- ammonium = NH4+ (weak acid)
- ammonia = NH3 (weak base)
- most ammonium is in weak acid form
Mechanism
- in PCT cells
- filtered glutamine is absorbed into PCT cells
- glutamine is then metabolised to NH4+ and bicarbonate
- NH4+ is secreted from PCT, mostly through NHE3 in exchange for sodium (ammonium ‘pretends’ to be H+)
- bicarbonate is reabsorbed
- downstream
- NH4+ is ionised and hence relies on ion transporters to move across the lumen
- NH4+ can substitute for K+ in NKCC transporters, and is reabsorbed in the thick ascending loop of Henle
- later in the collecting ducts, it diffuses back into the lumen as NH3 where it binds H+ secreted by intercalated cells to become NH4+ once more
- NH4+ is ionised and hence becomes trapped in the lumen, allowing excretion of large quantities of H+
Net effects
- for every ammonium molecule excreted, bicarbonate is created and reabsorbed
- binding of H+ in the distal nephron allows for the secretion of large amounts of H+
Response to acidaemia or alkalaemia
- acidaemia causes increased production of glutamine in the liver, leading to increased uptake in the PCT for use in the ammonium buffer system
- acidosis in the extracellular fluid of the PCT leads to increased glutamine absorption by PCT cells, augmenting this effect
- acid loads leads to increased type A intercalated cell activity, causing increased H+ secretion in the distal nephron and enhancing the downstream effects of the ammonium buffer
- the 'trapping' of NH4+ within the lumen is enhanced when urine is more acidic
- the opposite effects are seen with alkalaemia
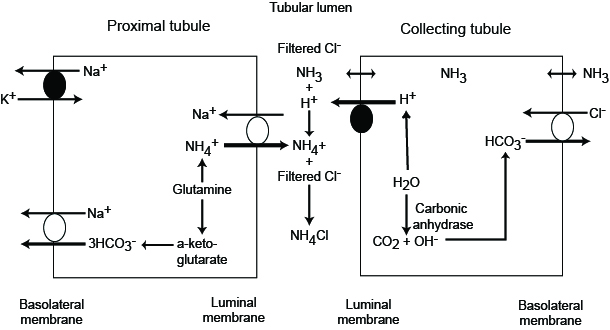
The ammonium buffer system
By eClinPath (Cornell University), licensed under CC-BY-NC-SA 4.0

