Stewart's physiochemical approach to acid-base chemistry
The Stewart approach provides a quantitative, physicochemical framework for understanding acid–base disturbances. It is a complex topic that relies on a few fundamental concepts.
Understanding Stewart's approach
Concept 1: Dependent Variables
In the Stewart approach, H⁺ and bicarbonate are considered dependent variables. This is because:
- the supply of H⁺ is virtually infinite due to the abundance of water
- bicarbonate is readily synthesised or converted to other substances in response to changes in H⁺, CO₂, or to maintain electroneutrality
Consequently, H⁺ and bicarbonate concentrations do not determine acid–base status; rather, they reflect the effects of other chemical processes in plasma.
Concept 2: Strong and weak ions
Strong ions are ions that dissociate completely.
Strong ions can be:
- strong cations - e.g. Na⁺, K⁺, Ca²⁺, Mg²⁺
- strong anions - e.g. Cl⁻, lactate⁻, ketones, SO4²⁻
Weak ions do not dissociate completely, existing in their ionised and unionised forms. They can be:
- weak anions - e.g.
- weak cations - e.g.
Weak cations exist in small concentrations, in part due to their tendency to bind to plasma proteins. In Stewart's approach, they are considered insignificant and ignored.
Concept 3: Weak anions are (mostly) weak acids
Virtually all weak anions come from weak acids (HA), which dissociate to H+ and a conjugate base (A-):
The most abundant weak acids are albumin and phosphates.
Bicarbonate is a weak anion that is the conjugate base of carbonic acid.
Concept 4: Electroneutrality
Plasma must maintain electrical neutrality.
Hence,
As discussed above, the concentration of weak cations is insignificant. We will ignore them.
Virtually all weak anions are the conjugate bases (A-) of acids (HA). Bicarbonate is integral to our understanding of the Stewart approach, so we consider it separately.
If we rearrange this we get:
The difference in concentration between strong cations and strong anions is known as the strong ion difference (SID).
Concept 5: Independent variables
There are four independent variables that influence H+ and HCO3- in serum:
- Strong ion difference (SID)
- Total weak acid concentration (ATOT)
- water
- CO2
Strong ion difference
Let's take our last equation and express it as a graph.
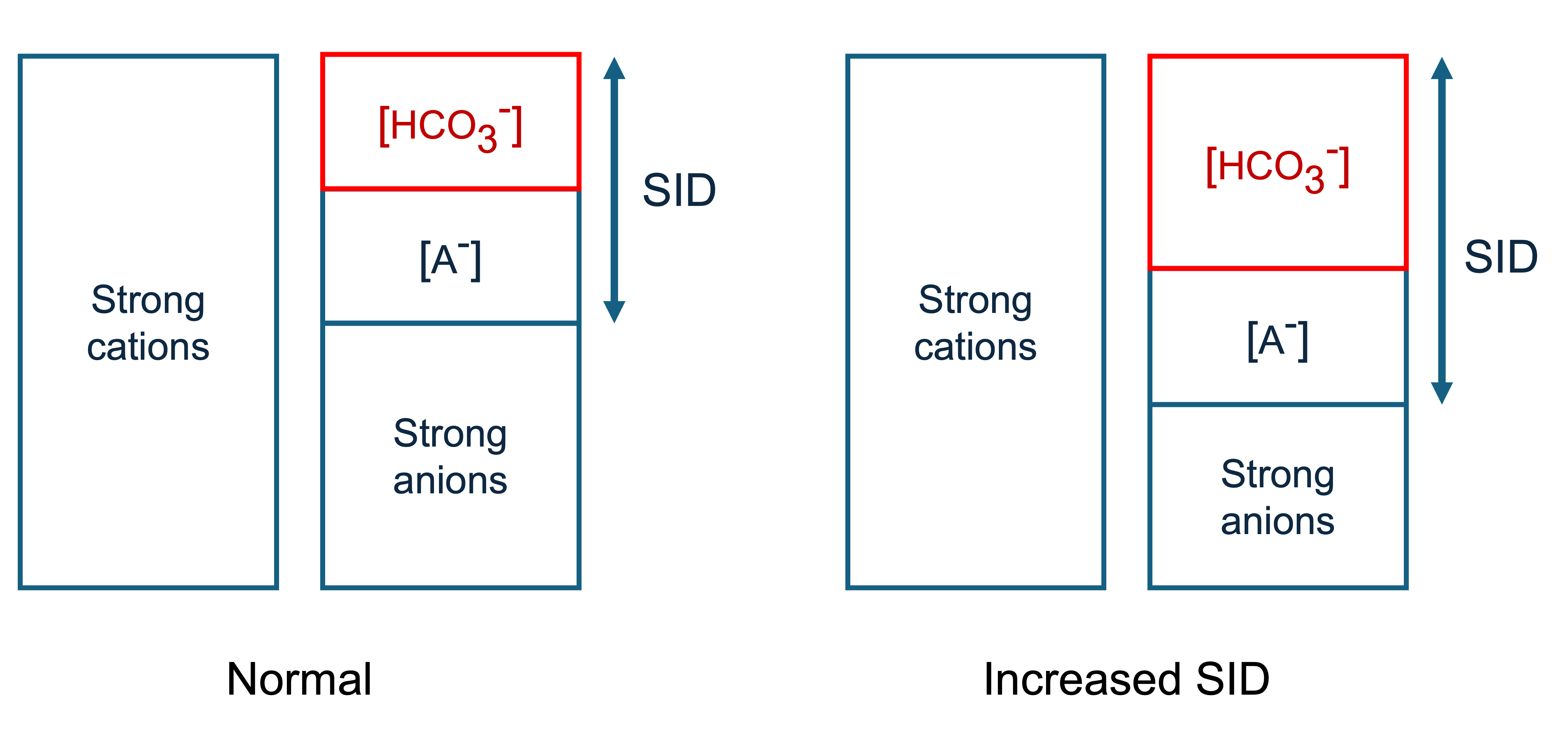
If we increase SID and leave A- the same, the bicarbonate concentration must increase to maintain electroneutrality. Therefore increasing SID causes a metabolic alkalosis. This could be done by increasing the strong cations or decreasing the strong anions.
Conversely, decreasing SID causes a metabolic acidosis. This could be done by decreasing the strong cations or increasing the strong anions (e.g. hyperchloraemia).
Of course, we don't actually measure all the ions when we draw blood from a patient. We only measure a few, the most abundant of which are Na+ and Cl-. As such, the calculation of SID at the bedside is often simplified.
A normal SID is 40-42mEq/L.
The concentrations used in these equations are in mEq/L, not mmol/L. The subtle difference is that we are measuring charge, not concentration. Ions that have a charge of 2 or more (e.g. calcium) count for double.
The SID of normal saline is 0 as the strong cations (Na+) and the strong anions (Cl-) are equal. Aggressive transfusion of normal saline will therefore decrease a patient's SID, causing a metabolic acidosis.
Total weak acid concentration
If we add weak acid to plasma, it will dissociate to form more of its conjugate base.
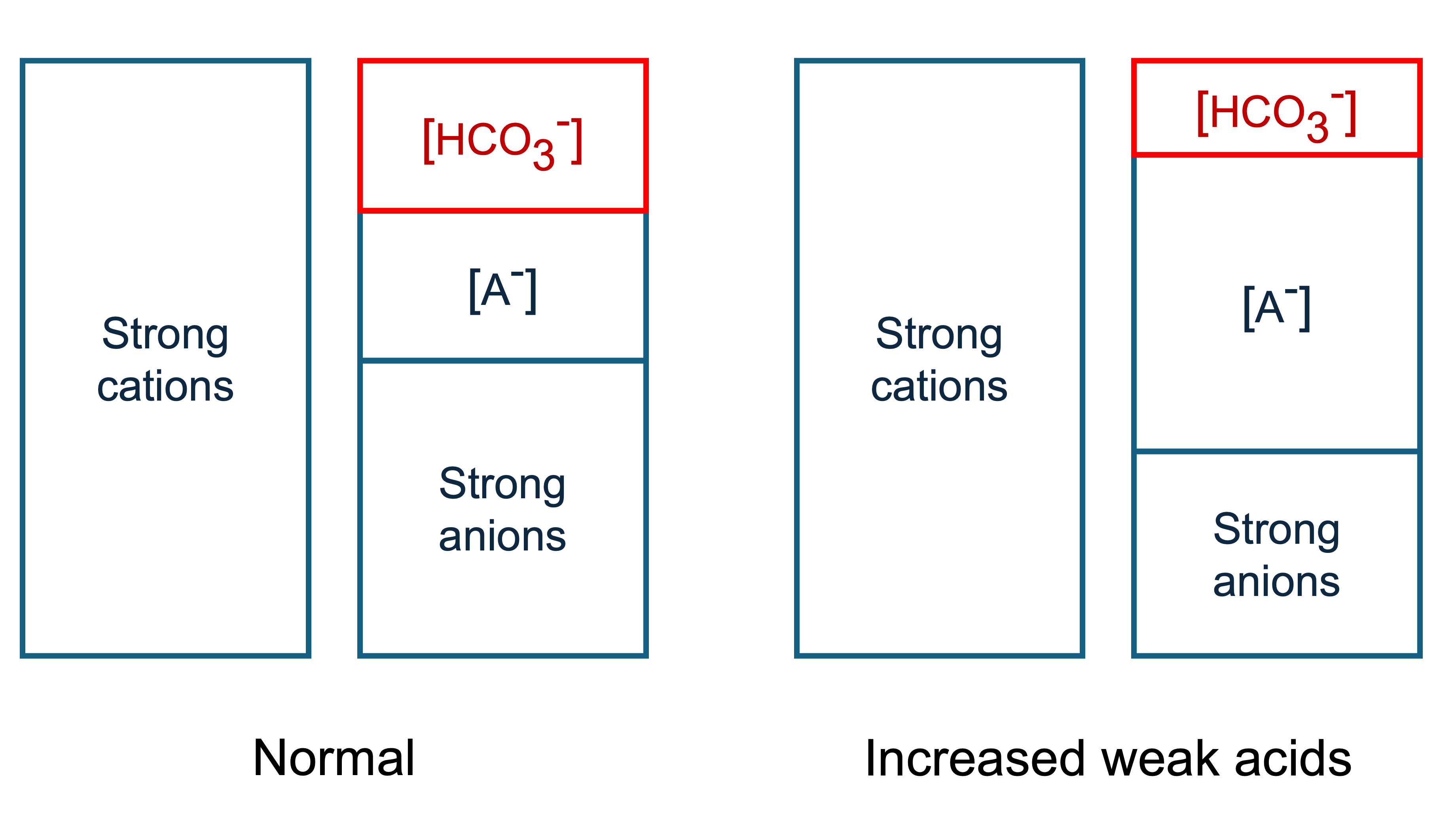
Referring to our graph again, we see that adding [A-] forces the bicarbonate concentration to fall to maintain electroneutrality.
Therefore adding weak acids causes a metabolic acidosis.
The total weak acid concentration is considered to be .
Water
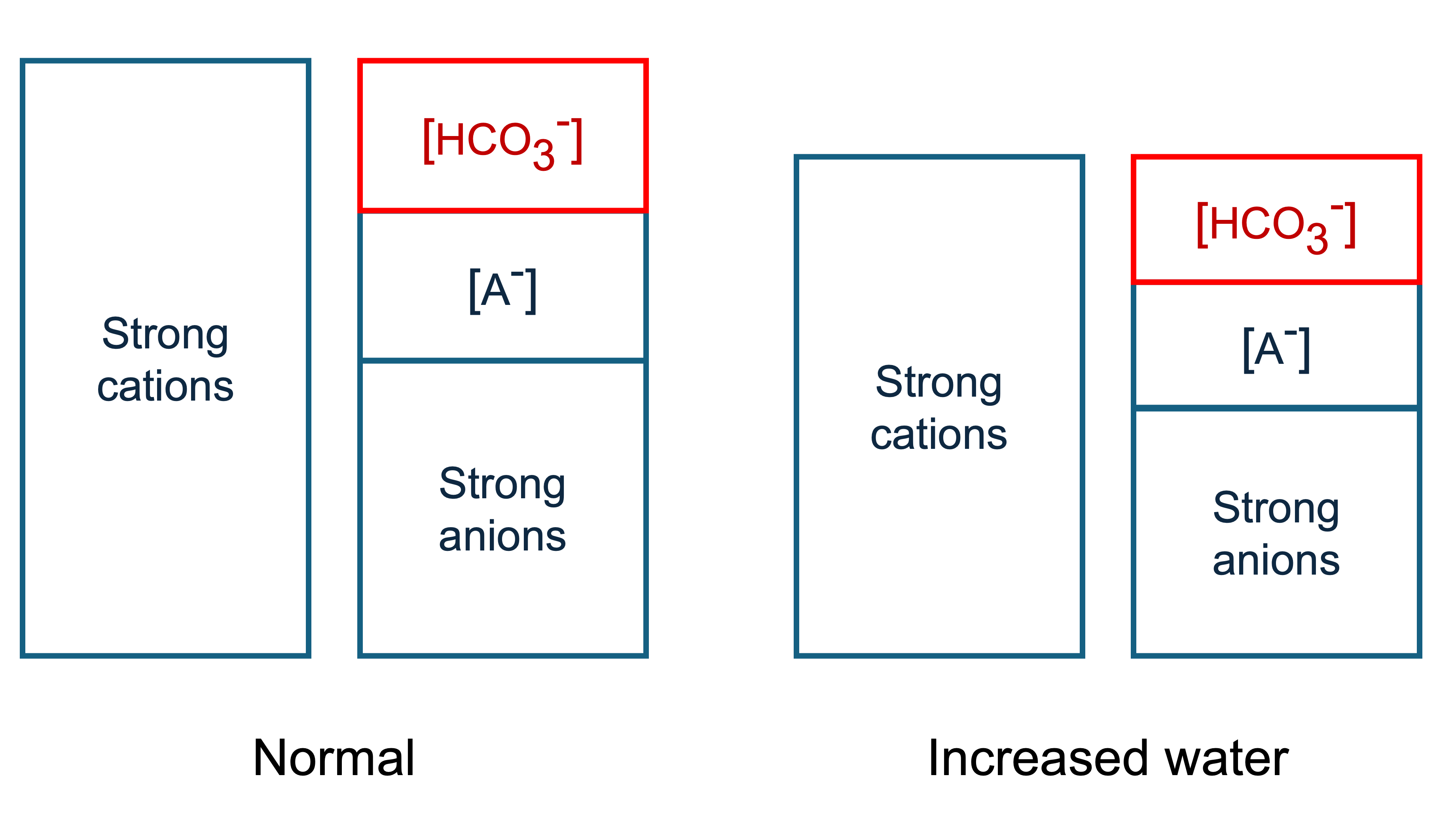
Adding water dilutes the concentration of everything in serum. This squishes everything on the graph downwards, and less bicarbonate is required to maintain electroneutrality.
Therefore adding water causes a metabolic acidosis, while hypovolaemia causes a metabolic alkalosis (contraction alkalosis).
CO2
The interpretation of CO2 is virtually the same as with the traditional approach.
Adding CO2 drives the equation to the right, generating H+ and producing a respiratory acidosis.
The exam answer
Purpose
Provides a quantitative physiochemical insight into acid-base pathology using the concept of electroneutrality.
Dependent variables
H+ and bicarbonate are dependent variables. Their concentrations are the result of interactions between acids and bases.
Independent variables
Independent variables influence H+ and bicarbonate concentrations:
- Strong ion difference, SID
- strong ions are ions that completely dissociate
- SID = strong cations - strong anions
- normal SID ~40-42 mEq/L
- examples
- strong cations: Na+, K+, Ca2+, Mg2+
- strong anions: Cl-, lactate, ketones, sulfate
- Total weak acid concentration,
- weak acids incompletely dissociate according to
- examples: albumin, phosphates
- Water
- pCO2
How they interact
In response to metabolic disturbances, the concentration of bicarbonate must change to maintain electroneutrality.
SID
- ↑SID causes ↑bicarbonate to maintain electroneutrality (metabolic alkalosis)
- e.g. due to increased strong cations (Na+)
- e.g. due to decreased strong anions (Cl-)
- ↓SID causes ↓bicarbonate (metabolic acidosis)
- e.g. due to decreased strong cations (Na+)
- e.g. due to increased strong anions (Cl-, lactate, ketones)
ATOT
- ↑ATOT causes ↓bicarbonate to maintain electroneutrality (metabolic acidosis)
- ↓ATOT causes ↑bicarbonate (metabolic alkalosis)
Water
- ↑water dilutes/decreases all components including bicarbonate (metabolic acidosis)
- ↓water concentrates/increases all components including bicarbonate (metabolic acidosis)
CO2
- ↑pCO2 drives this equation to the right, producing more H+ (respiratory acidosis)
- ↓pCO2 drives this equation to the left, decreasing H+ (respiratory alkalosis)
Benefits
- a quantitive approach which can unmask multiple competing metabolic disturbances
- can guide fluid resuscitation by choosing fluids with a more physiological strong ion difference
Limitations
- complex
- many components are not measured routinely
- not proven to improve outcomes

