Transport across cell membranes
Outline the passive transport mechanisms in the body
Movement of water
Osmosis
The passage of water from a compartment of low osmolality to one of high osmolality.
Water can pass slowly and directly through the phospholipid cell membrane or quickly via aquaporin channels.
Osmotic pressure
Osmotic pressure is technically defined as the pressure required to prevent the net movement of water across a semipermeable membrane due to a solute concentration difference.
In simpler terms, it is the force that moves water in the direction that will equilibrate a solute concentration gradient.
The rate of osmosis depends on the osmotic pressure gradient between the two compartments.
Osmotic pressure is given by the equation,
- = van ‘t Hoff index, which reflects how many particles a solute dissolves into
- = ideal gas constant
- = temperature in Kelvin
- = concentration of solute
Hence the osmotic pressure gradient is given by the equation, .
Diffusion refers to the movement of solute along its concentration gradient
Osmosis refers to the movement water to equilibrate a concentration gradient
Oncotic pressure is specifically the portion of osmotic pressure that is due to large impermeant particles (i.e. proteins). It is also known as colloid osmotic pressure.
Ultrafiltration
The movement of fluid across a membrane barrier (i.e. a capillary) along its pressure gradient, e.g. ultrafiltration of plasma at the glomerulus. The capillary acts as a molecular sieve, allowing small particles to pass with the fluid.
The rate of ultrafiltration is driven by the Starling forces.
In this diagram of a glomerular capillary, A represents fenestrations in the endothelium, B is the basement membrane, and C refers to the gaps between podocyte foot processes. These pores allow the passage of fluid via filtration.
Filtration barrier by Michal Komorniczak CC BY-SA 3.0 via Wikimedia Commons
Coffee filters work the same way as filtration at the capillary. Small, dissolved coffee particles are carried by water through the filter, leaving larger molecules behind as coffee grounds.
-
The movement of water in this example is via filtration.
-
The movement of coffee particles is via solvent drag.
-
The movement of the coffee as a whole is called bulk flow.
The ultra in ultrafiltration refers to fact that capillaries have tiny pores.
Movement of solute
Simple diffusion
Molecules diffuse across a membrane along an electrochemical or partial pressure gradient.
Lipid soluble compounds can diffuse across the phospholipid membrane.
- e.g. O2, CO2
- e.g. lipid soluble drugs (propofol, fentanyl)
Non-lipid soluble compounds generally require facilitated diffusion via channels.
Simple diffusion obeys Fick's laws of diffusion and the Gibbs-Donnan effect, which should be explained in an SAQ on this topic.
Facilitated diffusion
Carrier proteins in the cell membrane assist transport of a compound along its electrochemical gradient (i.e. via channels).
- e.g. reabsorption of glucose via passive GLUT2 channel on the basolateral membrane of the proximal convoluted tubule (PCT)
The rate of diffusion is dependent on Fick's laws of diffusion, the Gibbs-Donnan effect and the of the transporter.
- (transport maximum) is the point at which the maximum transport capacity of the channel is reached
- increasing the concentration gradient does not increase the rate of diffusion
- the Tmax of glucose in the PCT is approximately 350g/min (via SGLT1, SGLT2, and GLUT2 channels)
- if more than 350g/min of glucose is filtered at the glomerulus, the reabsorption capacity is exceeded, leading to glycosuria
Solvent drag
Refers to solute that is carried across the membrane during ultrafiltration.
- it occurs via paracellular movement rather than transcellular diffusion
- e.g. reabsorption of urea or K+ with water in PCT
Factors that increase solvent drag:
- anything that increases ultrafiltration
- greater concentration of solute in the original fluid
- smaller molecules are more likely to pass through the pores in the membrane
- greater sieving coefficient
Outline the active transport mechanisms in the body
Primary active transport
Energy is consumed to transport a substance against its electrochemical gradient.
Includes all ATPase pumps, for example:
- Na/K-ATPase
- H/K ATPase
- H-ATPase
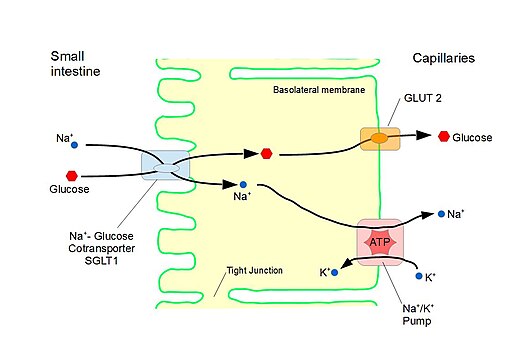
Secondary active transport
Primary active transport generates an electrochemical gradient which facilitates the movement of a different substance across the membrane. The movement of this second substance is via secondary active transport.
e.g. SGLT1 or SGLT2 channels in the proximal convoluted tubule (PCT)
- intracellular Na+ is low due to the action of Na/K-ATPase pumps
- SGLT1 and SGLT2 on the apical membrane reabsorb Na along this gradient, co-transporting glucose with it
Pinocytosis
The umbrella term for endocytosis and exocytosis. Refers to the movement of non-diffusible particles across membrane by packaging in vesicles.
Examples:
- reabsorption of insulin in PCT (endocytosis)
- transfer of IgG from mother to foetus across the placenta (endocytosis and exocytosis)
- catecholamine release (exocytosis)
- catecholamine reupteake (endocytosis)

